Which Family of Organic Molecules Is a Hydrocarbon?
3.7: Organic Compounds
All living things are formed generally of carbon compounds chosen organic compounds. The category of organic compounds includes both natural and constructed compounds that comprise carbon. Although a single, precise definition has yet to be identified by the chemistry community, most agree that a defining trait of organic molecules is the presence of carbon equally the principal element, bonded to hydrogen and other carbon atoms. However, some carbon-containing compounds such as carbonates, cyanides, and simple oxides (CO and CO2) are not classified as organic compounds.
Organic compounds are fundamental components of plastics, soaps, perfumes, sweeteners, fabrics, pharmaceuticals, and many other substances used daily. Organic compounds include compounds originating from living organisms and those synthesized by chemists. The existence of a wide assortment of organic molecules is a issue of carbon atoms' ability to form up to four strong bonds to other carbon atoms, resulting in bondage and rings of many different sizes, shapes, and complexities.
Hydrocarbons
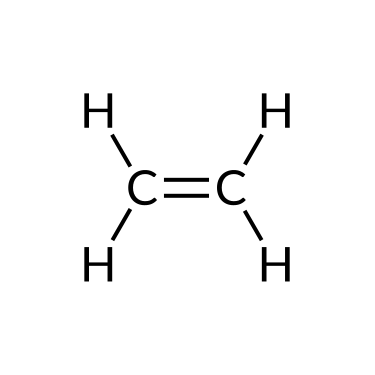
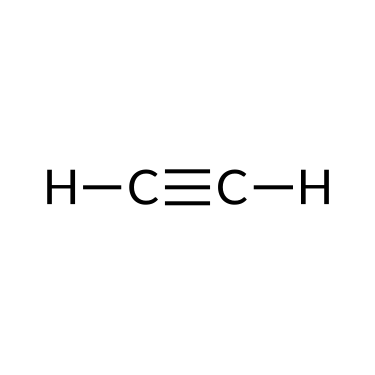
The simplest organic compounds contain only the elements carbon and hydrogen and are chosen hydrocarbons. Hydrocarbons may differ in the types of carbon-carbon bonds nowadays in their molecules. Those containing simply single bonds are called alkanes, while those containing double or triple bonds are alkenes and alkynes, respectively. Although all hydrocarbons are composed of just two types of atoms (carbon and hydrogen), there is a wide diverseness of hydrocarbons because they may consist of varying lengths of bondage, branched chains, and rings of carbon atoms, or combinations of these structures.
| | | |
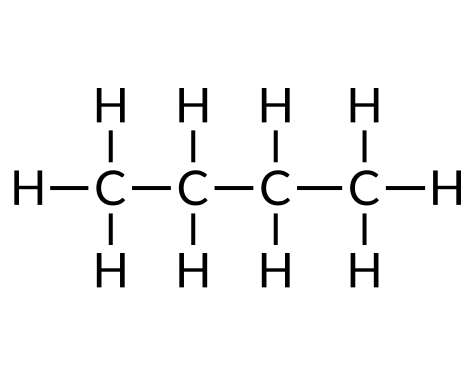
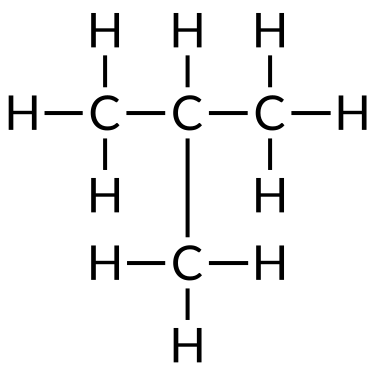
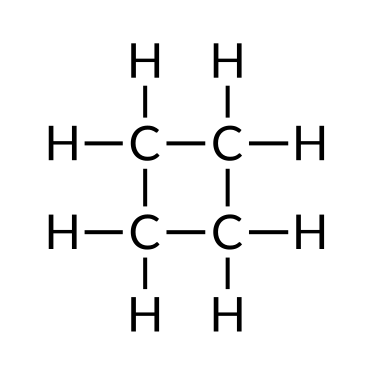
| Butane (C4H10) | Isobutane (CivHten) | Cyclobutane (C4Height) |
Hydrocarbons are used every day, mainly every bit fuels, such as natural gas, acetylene, propane, butane, and the primary components of gasoline, diesel fuel, and heating oil. Alkanes, or saturated hydrocarbons, comprise only unmarried covalent bonds between their carbon atoms. Properties such as melting point and the humid point usually change predictably equally the number of carbon and hydrogen atoms in the molecules alter.
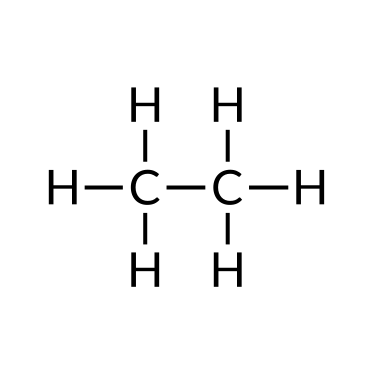
To name a simple paraffin, first identify the base name depending on the number of carbon atoms in the chain (meth = one, eth = two, prop = 3, but = 4, pent = five, hex = half-dozen, hept = 7, oct = viii, non = 9, and dec = ten). The base name is followed with a suffix — determined by whether the hydrocarbon is an alkane (-ane ), alkene (-ene ), or alkyne (-yne ). For example, a ii-carbon methane series is called ethane; a iii-carbon alkane is called propane; and a iv-carbon alkane is called butane. Longer chains are named as follows: pentane (5-carbon chain), hexane (six), heptane (vii), octane (viii), nonane (9), and decane (ten).
Alkenes and alkynes are unsaturated hydrocarbons containing double bonds and triple bonds, respectively, between at to the lowest degree two carbon atoms. Their nomenclature follows the same steps equally that of alkane series: base of operations proper noun + suffix. For example, a two-carbon alkene concatenation is called ethene, and a two-carbon alkyne is called ethyne; a three-carbon alkene is chosen propene, and a three-carbon alkyne is called propyne, and and then on.
| | | |
| Ethane (C2H6) | Ethene (CtwoH4) | Ethyne (CtwoH2) |
Functionalized Hydrocarbons
Incorporation of a functional group into carbon- and hydrogen-containing molecules leads to new families of compounds called functionalized hydrocarbons. The functional group is a feature atom or grouping of atoms that primarily determines the properties of hydrocarbon derivatives.
One blazon of functional grouping is the –OH group. Compounds that take an –OH functional group are alcohols. The name of the alcohol comes from the hydrocarbon from which it was derived. By convention, the hydrocarbon portion of the molecule is designated equally 'R'; so the general formula of an alcohol is R–OH. The concluding '–eastward' in the name of the hydrocarbon is replaced by '–ol'. In the instance of a branched alcohol, the carbon atom to which the –OH group is bonded is indicated by a number placed before the name. Other common functional groups are listed below. A group of compounds containing the same functional group forms a family unit.
| Family | Functional group | Instance | Formula | Name |
| Alcohols | | | C3HviiiO | Propanol |
| Ethers | | | C2H6O | Dimethyl ether |
| Aldehydes | | | C3H6O | Propanal |
| Ketones | | | C3H6O | Propanone (acetone) |
| Carboxylic acids | | | CiiiH6O2 | Propanoic acid |
| Esters | | | C4H8O2 | Ethyl acetate |
| Amines | | | C3H9N | Propylamine |
Ethers are compounds that contain the functional group –O–, with the general formula R–O–R'.
Another class of organic molecules contains a carbon atom continued to an oxygen atom by a double bond, commonly chosen a carbonyl group. The carbon in the carbonyl group tin attach to 2 other substituents leading to several subfamilies (aldehydes, ketones, carboxylic acids, and esters).
Functional groups related to the carbonyl group include the –CHO group of an aldehyde, the –CO– group of a ketone, the –CO2H group of a carboxylic acid, and the –COtwoR group of an ester. The carbonyl group, a carbon-oxygen double bond, is the key structure in these classes of organic molecules. Aldehydes contain at least one hydrogen atom fastened to the carbonyl carbon atom, ketones comprise two carbon groups attached to the carbonyl carbon atom, carboxylic acids contain a hydroxyl grouping attached to the carbonyl carbon atom, and esters contain an oxygen atom attached to another carbon group connected to the carbonyl carbon atom. All of these compounds contain oxidized carbon atoms relative to the carbon atom of an alcohol group.
The add-on of nitrogen into an organic framework leads to 2 families of molecules, namely amines and amides. Compounds containing a nitrogen atom bonded in a hydrocarbon framework are classified as amines. Compounds that take a nitrogen atom bonded to one side of a carbonyl group are classified as amides. Amines are a basic functional group. Amines and carboxylic acids tin combine in a condensation reaction to course amides.
This text is adapted from Openstax, Chemistry 2e, Section xx: Introduction, Openstax, Chemical science 2e, Section 20.1: Hydrocarbons, Openstax, Chemistry 2e, Section 20.two: Alcohols and Ethers, Openstax, Chemistry 2e, Section 20.ii: Aldehydes, Ketones, Carboxylic Acids, and Esters, and Openstax, Chemical science 2e, Section 20.2: Amines and Amides.
Source: https://www.jove.com/science-education/11255/organic-compounds
0 Response to "Which Family of Organic Molecules Is a Hydrocarbon?"
Enviar um comentário